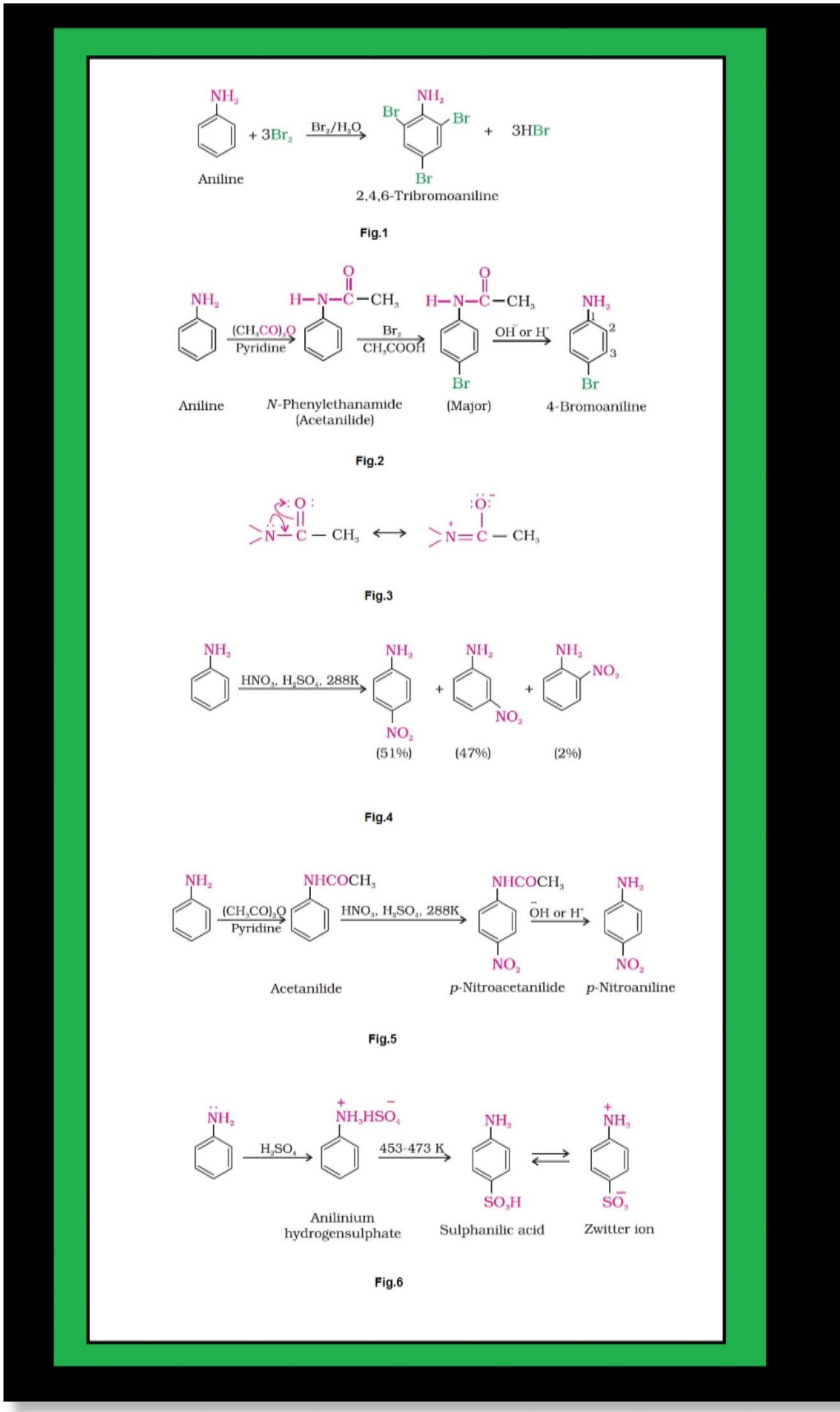
Basic character of amines :
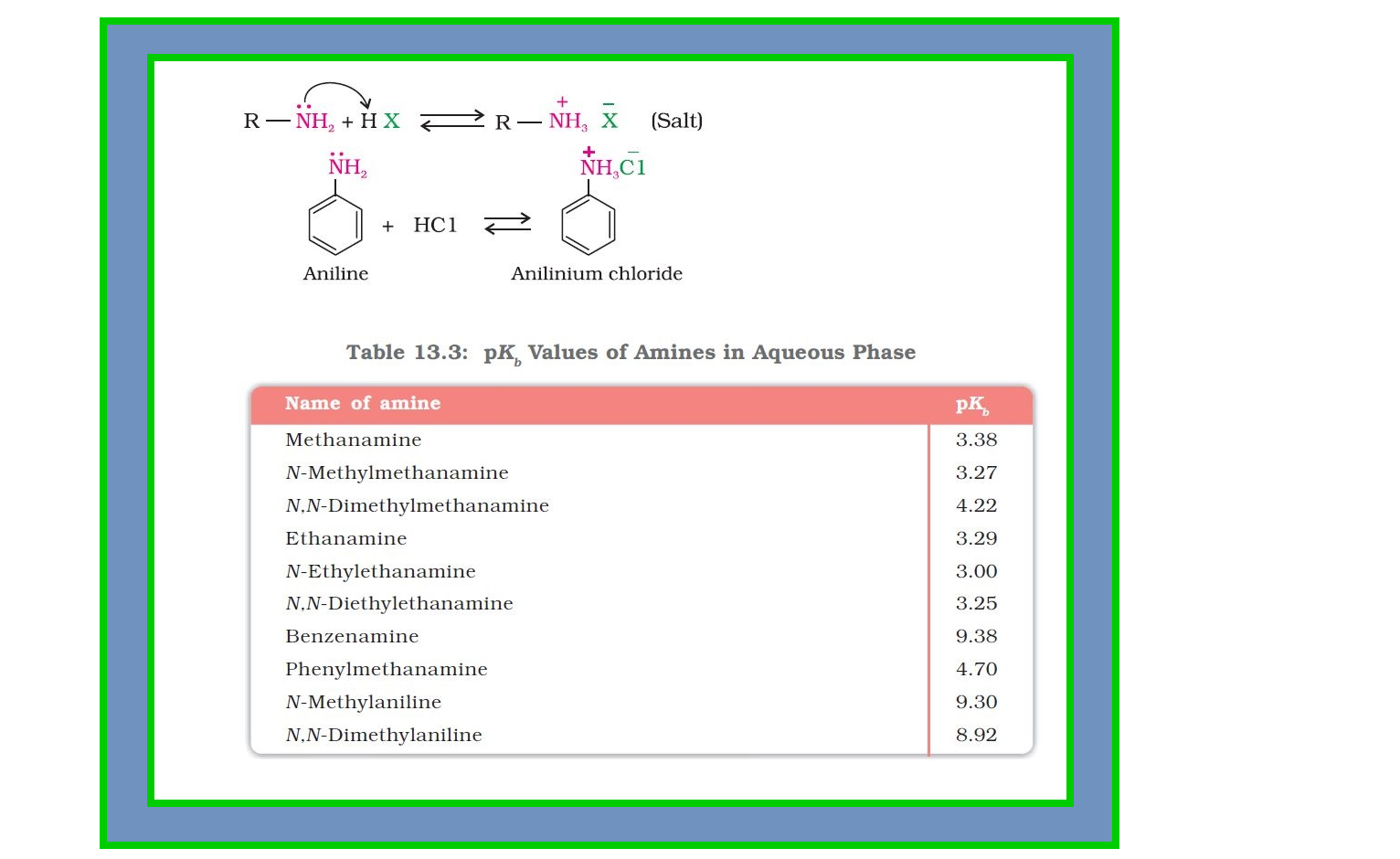
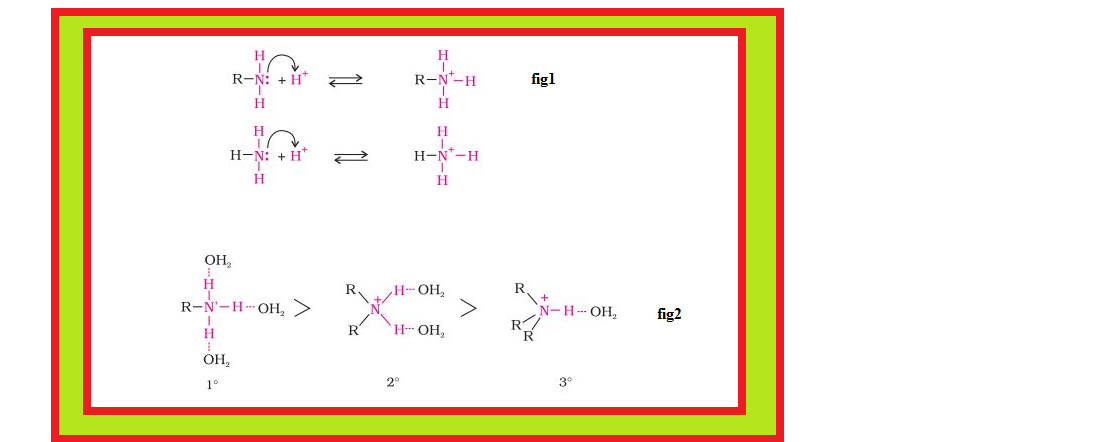
`=>` Amines, being basic in nature, react with acids to form salts. See fig.
`=>` Amine salts on treatment with a base like `color{red}(NaOH)`, regenerate the parent amine.
`color{red}(R overset(+)NH_3 overset(-)X + overset(-)OH →R overset(* *)N H_2+H_2O + overset(-)X)`
`=>` Amine salts are soluble in water but insoluble in organic solvents like ether.
● This reaction is the basis for the separation of amines from the non basic organic compounds insoluble in water.
`=>` The reaction of amines with mineral acids to form ammonium salts shows that these are basic in nature. Amines have an unshared pair of electrons on nitrogen atom due to which they behave as Lewis base.
● Basic character of amines can be better understood in terms of their `color{red}(K_b)` and `color{red}(pK_b)` values as explained below :
`color{red}(R - NH_2 +H_2O ⇄ R - overset(+)N H_3+ overset(-)OH)`
`color{red}(K = ( [ R- overset(+)NH_3] [O overset(-)H])/([R - NH_2] [H_2O]))`
or `color{red}(K[H_2O] = ([R - overset(+)NH_3][overset(-)OH])/([R-NH_2]))`
or `color{red}(K_b = ( [ R - overset(+)N H_3] [ overset(-)O H])/([R- NH_2]))`
`color{red}(pK_b = - log K_b)`
`=>` Larger the value of `color{red}(K_b)` or smaller the value of `color{red}(pK_b)`, stronger is the base.
`=>` The `color{red}(pK_b)` values of few amines are given in Table 13.3.
`=>` `color{red}(pK_b)` value of ammonia is `4.75`.
`=>` Aliphatic amines are stronger bases than ammonia due to `+I` effect of alkyl groups leading to high electron density on the nitrogen atom.
● Their `color{red}(pK_b)` values lie in the range of `3` to `4.22`.
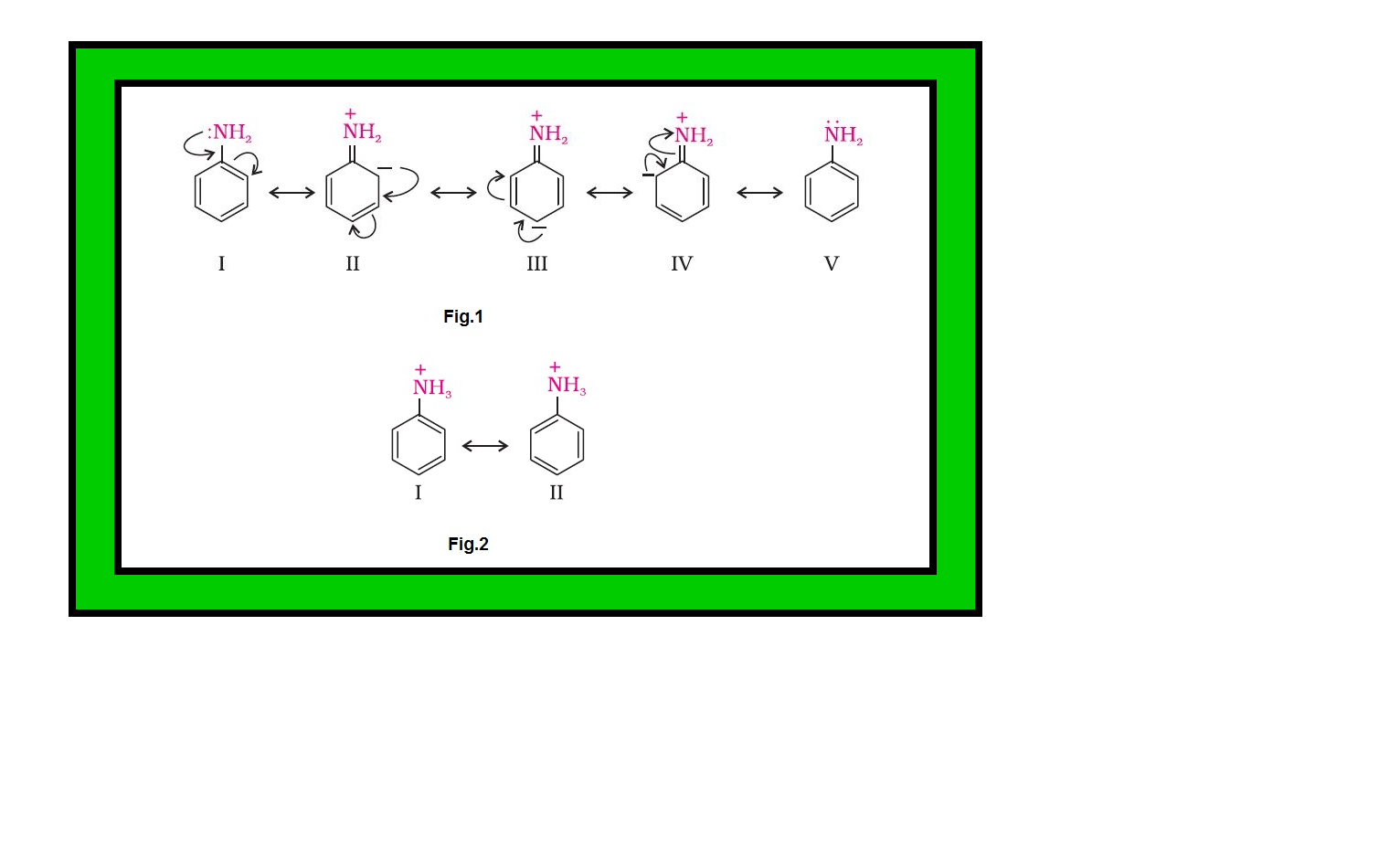
`=>` On the other hand, aromatic amines are weaker bases than ammonia due to the electron withdrawing nature of the aryl group.
`=>` You may find some discrepancies while trying to interpret the `color{red}(K_b)` values of amines on the basis of `color{red}(+I)` or `color{red}(–I)` effect of the substituents present in amines.
`=>` Besides inductive effect, there are other effects like solvation effect, steric hinderance, etc., which affect the basic strength of amines.
`=>` Amine salts on treatment with a base like `color{red}(NaOH)`, regenerate the parent amine.
`color{red}(R overset(+)NH_3 overset(-)X + overset(-)OH →R overset(* *)N H_2+H_2O + overset(-)X)`
`=>` Amine salts are soluble in water but insoluble in organic solvents like ether.
● This reaction is the basis for the separation of amines from the non basic organic compounds insoluble in water.
`=>` The reaction of amines with mineral acids to form ammonium salts shows that these are basic in nature. Amines have an unshared pair of electrons on nitrogen atom due to which they behave as Lewis base.
● Basic character of amines can be better understood in terms of their `color{red}(K_b)` and `color{red}(pK_b)` values as explained below :
`color{red}(R - NH_2 +H_2O ⇄ R - overset(+)N H_3+ overset(-)OH)`
`color{red}(K = ( [ R- overset(+)NH_3] [O overset(-)H])/([R - NH_2] [H_2O]))`
or `color{red}(K[H_2O] = ([R - overset(+)NH_3][overset(-)OH])/([R-NH_2]))`
or `color{red}(K_b = ( [ R - overset(+)N H_3] [ overset(-)O H])/([R- NH_2]))`
`color{red}(pK_b = - log K_b)`
`=>` Larger the value of `color{red}(K_b)` or smaller the value of `color{red}(pK_b)`, stronger is the base.
`=>` The `color{red}(pK_b)` values of few amines are given in Table 13.3.
`=>` `color{red}(pK_b)` value of ammonia is `4.75`.
`=>` Aliphatic amines are stronger bases than ammonia due to `+I` effect of alkyl groups leading to high electron density on the nitrogen atom.
● Their `color{red}(pK_b)` values lie in the range of `3` to `4.22`.
`=>` On the other hand, aromatic amines are weaker bases than ammonia due to the electron withdrawing nature of the aryl group.
`=>` You may find some discrepancies while trying to interpret the `color{red}(K_b)` values of amines on the basis of `color{red}(+I)` or `color{red}(–I)` effect of the substituents present in amines.
`=>` Besides inductive effect, there are other effects like solvation effect, steric hinderance, etc., which affect the basic strength of amines.